Nuvaxovid
As of January 2022 approximately 300 million people worldwide have been infected with the severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 that causes coronavirus. Esimerkiksi aiemmin sairastettu koronavirustauti ei estä rokotuksen antamista.
Novavax Nuvaxovid Gets Expanded Conditional Marketing Authorization In Eu For Use As Booster For Adults Aged
The vaccine is safe and effective for all individuals aged 18 and above.

. Folkhälsomyndigheten rekommenderar att det proteinbaserade covid-19-vaccinet Nuvaxovid inte ges till personer som är 30 år och yngre. What are subunit vaccines. Since 1986 use this technology.
Det eftersom att data från Australien gett signaler. Nuvaxovid is authorised in Northern Ireland under the CMA granted by the European Medicines Agency on 20 December 2021. The addition of the saponin-based.
Nuvaxovid vaccine pause for young people justice system spending Västerås shooting young women have more debt than 10 years ago. Nu stoppar Folkhälsomyndigheten användningen bland personer som är 30. Rokotteesta ei myöskään ole haittaa vaikka.
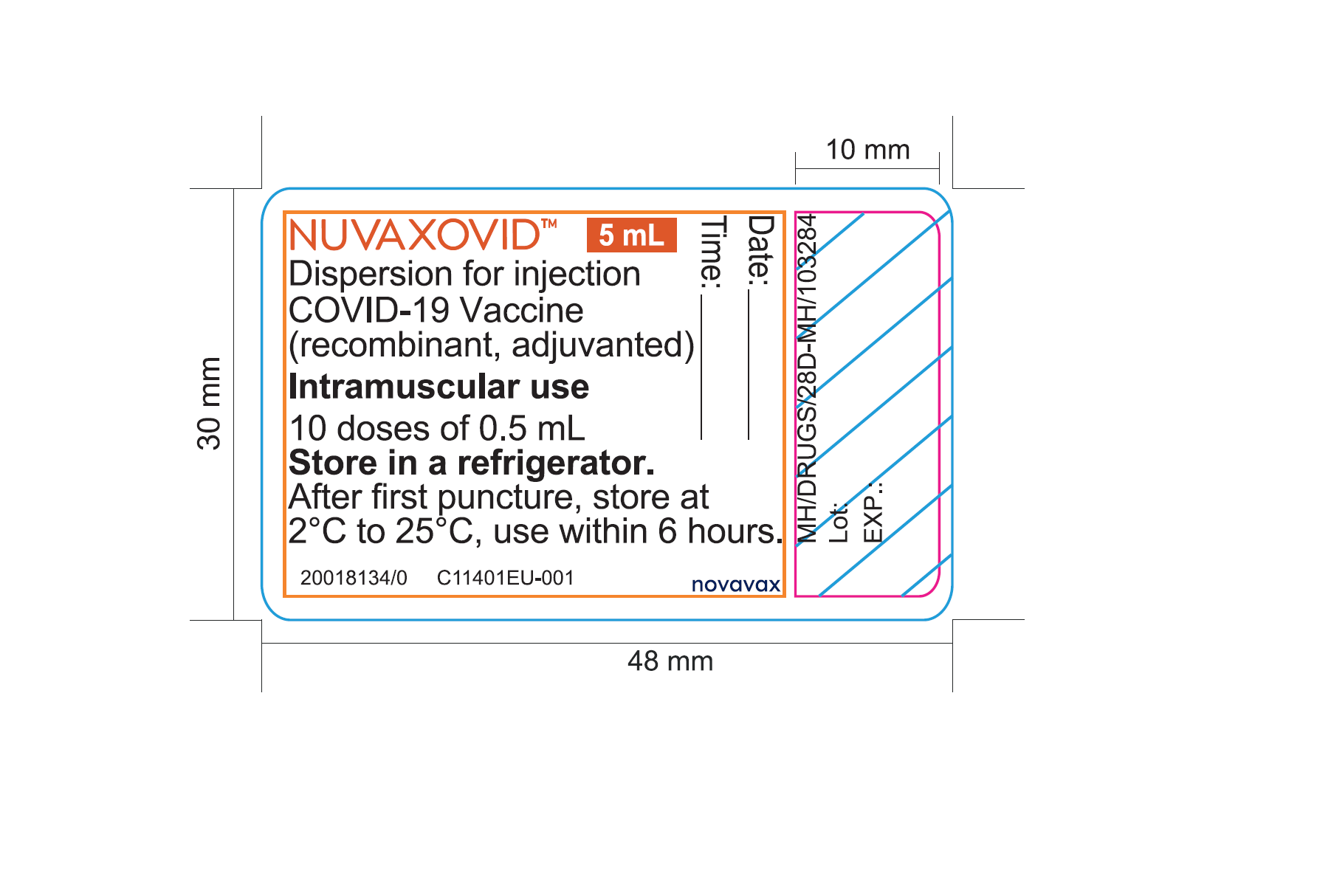
The addition of the saponin-based Matrix-M adjuvant. About Nuvaxovid NVX-CoV2373 Nuvaxovid is a protein-based vaccine engineered from the genetic sequence of the first strain of SARS-CoV-2 the virus that causes. Nuvaxovid is packaged as a ready-to-use liquid formulation in a vial containing ten doses.
Nuvaxovid is a vaccine for preventing coronavirus disease 2019 COVID-19 in people aged 12 years and older. Nuvaxovid is composed of purified full length severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 recombinant spike S protein that is stabilised in its prefusion conformation. The Nuvaxovid NVX-CoV2373 Novavax vaccine is a recombinant spike S protein nanoparticle vaccine combined with the Matrix-M adjuvant.
1 day agoDet proteinbaserade covid-19-vaccinet Nuvaxovid ska inte ges till personer som är 30 och yngre meddelar Folkhälsomyndigheten. Nuvaxovid-rokote sopii lähes kaikille aikuisille. 1 day ago3rd November 2022 0355 GMT11.
The vaccination regimen calls for two 05 ml doses 5 mcg antigen and 50 mcg Matrix-M adjuvant given. 1 day agoSverige Covid-19-vaccinet Nuvaxovid skulle erbjudas till personer som var tveksamma till vaccinationen. Data från Australien pekar mot en ökad.
In line with the WHO Prioritization Roadmap and the WHO Values Framework older adults health workers. This CMA has similar requirements to that. Nuvaxovid is composed of purified full-length SARS-CoV-2 recombinant spike S protein that is stabilised in its prefusion conformation.
About 14m doses of the Nuvaxovid vaccine developed by the US biotech company Novavax are to arrive in Germany this week the countrys health minister Karl Lauterbach. Beslutet är temporärt och gäller från. Company Novavax should not be given to individuals.
2 Xinhua -- Nuvaxovid the COVID-19 vaccine created by US. Clinical trials showed that beginning 1 week after the second dose Novavax Nuvaxovid COVID-19 vaccine was. Nuvaxovid is composed of purified full-length SARS-CoV-2 recombinant spike S protein that is stabilised in its prefusion conformation.
The Nuvaxovid vaccine a protein-based vaccine engineered from the genetic sequence of the first strain of the SARS-CoV-2 virus which causes COVID-19. 90 effective in protecting trial participants aged 18 and above against COVID. Nuvaxovid contains a version of a protein found on the.
This vaccine called Nuvaxovid produced by the company Novavax is a so-called subunit vaccine and differs from the previously approved vaccines. As at 31 May four cases of adverse reactions were reported out of the 2792 doses of Nuvaxovid administered here at a 014 per cent incidence rate the report published on. 1 day agoBakgrunden till beslutet är signaler om ökad risk för hjärtmuskelinflammation myokardit och hjärtsäcksinflammation perikardit.
The proteins inside the Novavax vaccine. The flu vaccine and the hepatitis B vaccine which doctors have administered throughout the US.
Distribution Of Nuvaxovid With English Only Vial And Carton Labels Canada Ca
Nuvaxovid Gets Expanded Provisional Approval In Nz As Covid 19 Booster For Adults

Novavax Covid 19 Vaccine Nuvaxovid Data On Side Effects

Novavax Stock Looks Like A Good Value With Its New Combined Vaccine
Who Lists 10th Covid 19 Vaccine For Emergency Use Nuvaxovid Strategic Partnership For Health Security And Emergency Preparedness Sph Portal

Nuvaxovid Novavax Covid 19 معلومات در باره واکسین Australian Government Department Of Health And Aged Care
Faq What You Need To Know About Novavax S Non Mrna Covid 19 Vaccine Nuvaxovid Cna

Takeda Gains Approval In Japan For Nuvaxovid Covid 19 Vaccine For Prim
Novavax Makes One Million Doses Of Nuvaxovid Available For Use In The United Kingdom Pharmtech Focus
Fda To Authorize Novavax S Covid 19 Vaccine Politico

Novavax Nvx Cov2373 Nuvaxovid In Europe And Australia Covovax In India And The Philippines Tak 019 In Japan
Novavax Covid Vaccine Nuvaxovid Gets Provisional Nod In New Zealand For Adolescents Aged 12 Through 17

Fda Advisers Overwhelmingly Endorse Novavax Covid 19 Vaccine Ars Technica

Novavax Nuvaxovid Covid 19 Vaccines Will Also Be Available From Rauma Healthcare Services In The Future Rauma Fi

Novavax Nuvaxovid Covid 19 Vaccine Star Pharmacy
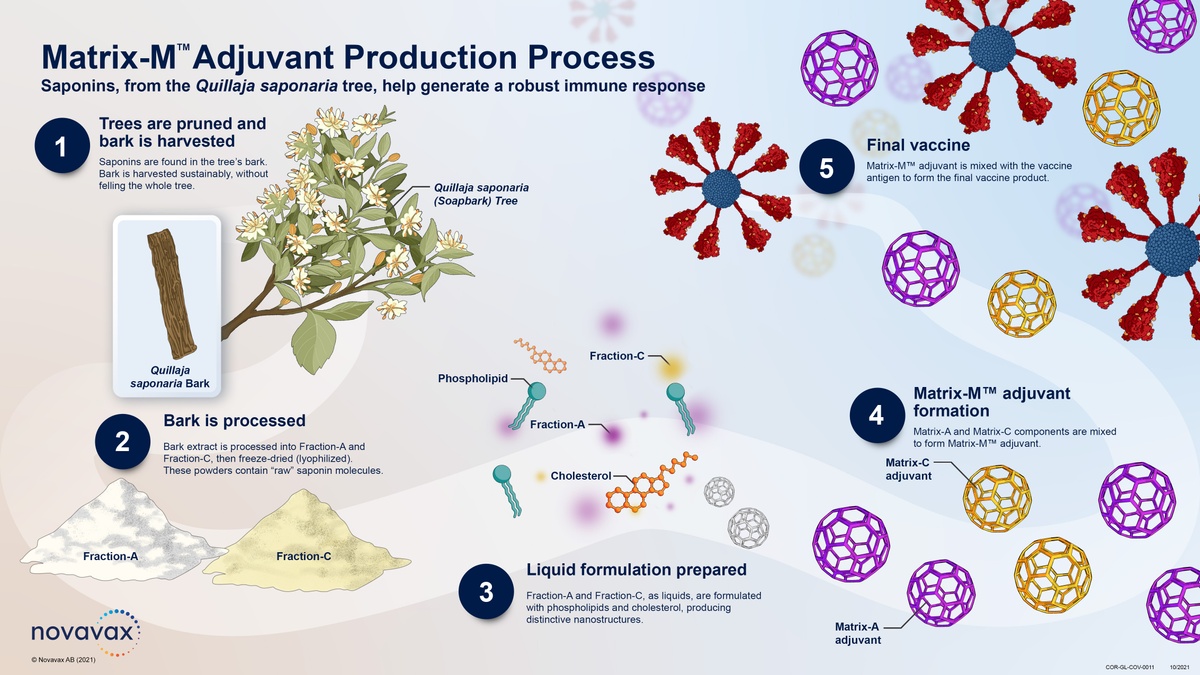
Novavax European Commission Grants Conditional Marketing Authorization For Novavax Covid 19 Vaccine

Canadian Trademarks Details Nuvaxovid 2163962 Canadian Trademarks Database Intellectual Property And Copyright Canadian Intellectual Property Office Innovation Science And Economic Development Canada
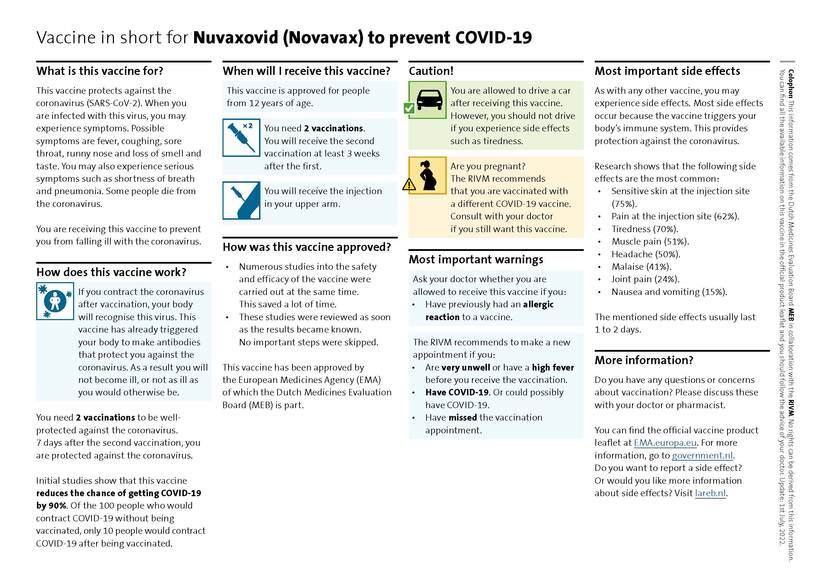
Vaccine In Short For Nuvaxovid Novavax Publication Medicines Evaluation Board
Tga Investigates Possible Myocarditis Link To Nuvaxovid Ausdoc